Optimizing Mammogram Screenings: A Genetic Approach to a Personalized Screening Schedule
January 04, 2024

© Arthon Meekodong via Canva.com
Breast cancer screening has long been a cornerstone of women's healthcare. With 1 in 8 women diagnosed with breast cancer in their lifetime1, the United States Preventive Services Task Force (USPSTF) has developed screening recommendations to help detect early-stage cancer. Notably in 2023, the USPSTF revised the recommended age for biennial mammogram screenings for women with average risk to start at age 40 instead of 502, estimated to result in 19% more lives being saved3 by starting screening earlier. While initiating screening at an earlier age offers advantages to a wide demographic, concerns about the potential of over-screening prompted research into the feasibility of identifying women with lower breast cancer risk who could safely delay mammograms. While guidelines address high-risk individuals, a notable gap exists in providing recommendations tailored to those at lower risk.
To gain insight into a patient's risk level, physicians are able to utilize genetic testing to understand an individual's genetic makeup, providing precise insights into their predisposition to various health conditions, including breast cancer. Armed with this genetic information, healthcare providers could craft tailored screening strategies that align with an individual’s specific risk profile. This genetic risk-based approach underscores the value of genetics in individualizing the onset of screening to help avoid over-screening and its associated costs. Surprisingly, genetic information is not currently being widely utilized to identify women at risk of breast cancer or other diseases in clinical practice, despite its potential to make a significant positive impact for patients.
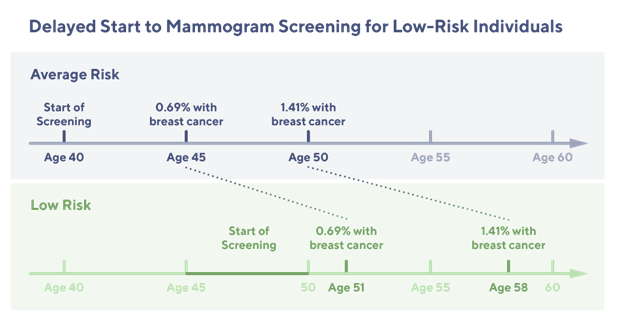
A recent retrospective analysis of 25,591 women from the Healthy Nevada Project4 sheds light on the potential benefits of this genetic risk-based approach. The study classified 2,338 (9.1%) of these women as having a low genetic risk for breast cancer. What's remarkable is that these women exhibited a significantly lower and later onset of breast cancer compared to their average or high-risk counterparts. This finding suggests that it might be safe for low-risk women to delay mammogram screening by 5 to 10 years without compromising their health.
These findings from the Healthy Nevada Project study challenge the USPSTF’s one-size-fits-all approach to screening. Delaying mammogram screening for individuals at low-risk of breast cancer could not only reduce healthcare costs and optimize healthcare resource allocation but also alleviate the unnecessary anxiety that can accompany frequent screenings.
Genetic screening can also enable identification of individuals that are at high risk and should undergo mammography screening at an earlier age and with greater frequency5. A focus on risk may reenforce the importance of preventative breast cancer screening. However, disparities pervade all aspects of breast cancer screening and treatment. Current clinical risk assessments are often inconsistently applied, leading to inequitable outcomes. Given the current limitations of existing screening methods to identify at-risk individuals, an alternative approach to identifying individuals' risk levels is to adopt a population-level genetic screening approach.
A population-wide approach can make healthcare more personalized and efficient. Rather than subjecting all women to the same screening schedule, we can tailor screening recommendations to an individual's unique genetic profile. This approach not only ensures that high-risk individuals receive the attention they need earlier but also reduces the burden on the healthcare system by enabling low risk individuals to defer preventative screening.
Implementing a population-wide approach that tailors healthcare to each patient is not only an effective strategy for improving healthcare outcomes but is also increasingly cost-effective for health systems and the industry as a whole. As the healthcare landscape continues to evolve, advances in technology and data analysis have significantly reduced the costs associated with personalized medicine approaches. Recent studies6 have demonstrated the economic viability of population-wide screening initiatives. These findings underscore that by identifying and addressing health risks at an early stage, we can mitigate the financial burden of treating advanced diseases while simultaneously improving patient outcomes. In essence, the upfront investment in personalized population health measures ultimately translates into substantial cost savings and a more efficient healthcare system in the long run.
The current approach to breast cancer screening, while widely practiced, stands to benefit significantly from the incorporation of genetic information to tailor screening strategies. This personalized approach, grounded in assessing individual genetic risk, offers a promising pathway to enhance patient well-being, reduce healthcare costs, and optimize the use of resources, which is particularly crucial in the evolving post-pandemic healthcare environment.
Beyond breast cancer screening, the potential of genetic information in healthcare is far-reaching. It offers critical insights into a variety of diseases, including cancer, genetic disorders, and cardiovascular diseases, allowing for early intervention and tailored treatments. Integrating genetic data into regular medical practice enables providers to offer more precise, effective care, enhancing patient outcomes. This marks a significant shift towards a more efficient and equitable healthcare system in the era of personalized medicine.
Sources:
- American Cancer Society. (2023). Breast cancer statistics.
- U.S. Preventive Services Task Force. (2023). Breast cancer: Screening.
- U.S. Preventive Services Task Force. (2023). Draft recommendation statement: Breast cancer: Screening.
- Bolze, Alexandre PhD; Elizabeth T. Cirulli, PhD; Catherine Hajek, MD1; et al (2023). The Potential of Genetics in Identifying Women at Lower Risk of Breast Cancer. doi:10.1001/jamaoncol.2023.5468
- Schuller, A., Krapcho, M., & Van Doren, M. (2023). Systematic Review and Meta-Analysis of the Relationship Between Breast Cancer Risk and Use of Exogenous Estrogen. medRxiv, 2023.05.17.23290132v1. doi:10.1101/2023.05.17.23290132v1
- DeBoer, M. D., & Paskett, E. C. (2023). Cost-Effectiveness of Population Screening for CDC Tier 1 Conditions. Preventive Medicine, 177, 107073.