Search
Results for 'doctors'
Clear-
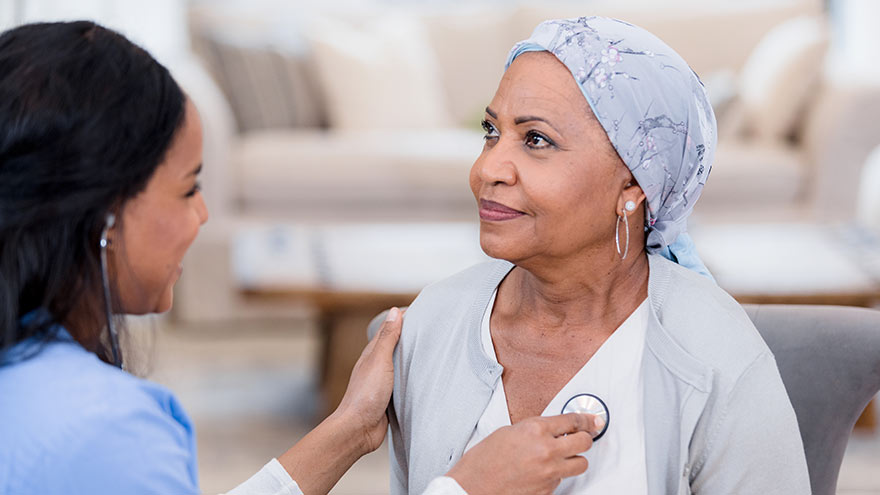
Ovarian Cancer Survivor Shares Decision to Try Clinical Trial
While there used to be three basic treatment options for cancer -- surgery, radiation and chemotherapy, or a combination of the three -- there's a fourth option: clinical trials. Here, a Renown patient shares her successful battle with ovarian cancer, aided by a clinical trial. Shari Flamm's battle with ovarian cancer began in 2011. She was experiencing prolonged bleeding, irregular thyroid levels and anemia and was scheduled to undergo a hysterectomy. Before the surgery, her gynecologist ran routine tests to check for cancer as a precautionary measure. All tests were negative for cancer, expect her CA 125 test. A CA 125 test measures the amount of the protein CA 125 (cancer antigen 125) in the blood. In some cases, a CA 125 test may be used to look for early signs of ovarian cancer in women with a very high risk of the disease. In most laboratories, the normal level is 0 to 35 units/ml. Flamm's CA 125 level was 121. As Flamm can attest, early diagnosis played a key role in her battle with ovarian cancer. September is Gynecologic Cancer and Ovarian Cancer Awareness Month – an important time to learn the signs, symptoms and risk factors of this type of cancer so your doctor can diagnosis the disease as early as possible. Ovarian Cancer: Round One Despite the elevated CA 125 results, her doctor recommended they move forward with the hysterectomy. But when surgery began, doctors discovered a mass. She had stage 4 cancer. The procedure was halted, the mass was biopsied and she was immediately seen by Dr. Peter Lim of the The Center of Hope. Following diagnosis, Flamm underwent surgery with Dr. Lim to remove the cancer, which had spread to part of diaphragm, spleen, colon and other organs. Three months after surgery, Flamm had recovered enough to start six rounds of chemotherapy in her hometown of Carson City. She continued working at a doctor's office during her treatment, and was grateful for Dr. Lim’s ability to co-manage her care so she could stay close to work and family. “To me, chemo was the scariest part because I didn’t like feeling sick,” Flamm says. Thankfully, her body responded well to the treatments and she was back to the things she loved. “I stated working out at the gym, even if it was only for 10 minutes,” she says. She also stayed positive by spending time with her grandchildren, attending a San Jose Sharks hockey game, going for walks and enjoying concerts. Ovarian Cancer: Round Two In November 2014, Flamm had a cancer check-up. That’s when doctors discovered three cancerous tumors. For this round, Flamm choose another treatment option -- clinical trials at Renown Institute for Cancer. Clinical trials are the studies that test whether drugs work, and inform doctors' decisions about how to treat their patients. Flamm participated in a clinical trial that featured oral-targeted therapy stronger than IV chemotherapy. The hope was for the drug to shrink her tumors, however the result was stabilization -- meaning the lumps weren’t growing or spreading. The best part of the clinical trial, Flamm says, was the constant monitoring. Between the CT scans every six weeks, a heart scan every three months and monthly doctor visits, she was confident that if the cancer started growing or spreading, her healthcare team would catch it right away. For Flamm, the benefits of the clinical trial included less hair loss, less fatigue and more time to focus on what’s important in her life -- her family. “I decided I wasn’t going to be that sick grandma on the couch with cancer,” Flamm says. After taking the oral medication for one year, Flamm developed a rash and discontinued treatment due to discomfort. Clinical Trials, Setbacks and Survival In June 2016, two of the three tumors began to grow and had to be surgically removed. Despite the setback, Flamm was determined to maintain a positive outlook. "You have to stay positive because cancer feeds off anger, depression and stress," Flamm says. Flamm was released to go home with clear margins, meaning the tumors were removed and are surrounded by a rim of normal tissue that does not have cancerous cells. Flamm says her outlook on life has changed drastically since her first cancer diagnosis. “Your whole mentality changes when cancer disturbs your life," Flann says. "The things that weren’t important, are now ever so important. I’m a lot calmer now,” Flamm says.
Read More About Ovarian Cancer Survivor Shares Decision to Try Clinical Trial
-
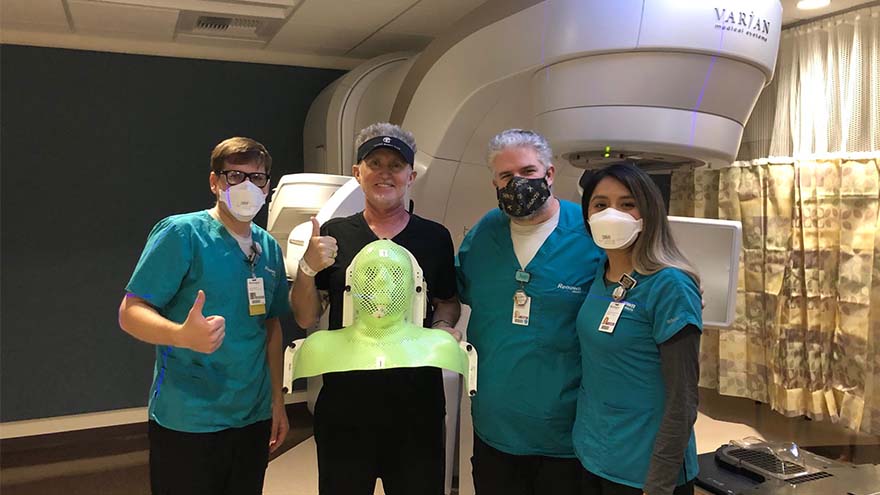
A Cancer Diagnosis and a Move to Reno
Michael Millman was all set to move to Reno from the Bay Area when he noticed a pimple-like growth on his forehead, and he decided to get biopsied "just in case." It was July 2020, less than six months into the COVID-19 pandemic, when Michael got the call that the biopsy came back cancerous. He was in shock. Still living in the Bay Area at the time, he immediately scheduled to have the basal cell carcinoma removed in August. After the removal, he thought he was in the clear, but a few months later, Michael noticed that his lymph nodes felt weird, and he even cut himself shaving because of some persistent swelling in the area. Given his recent history of skin cancer, Michael immediately scheduled an appointment with a specialist in the Bay Area. "I met with an ear, nose and throat doctor who suggested a fine needle biopsy of my lymph nodes, tongue and an MRI, both with and without contrast," Michael said. "I remember feeling dreadful and that I couldn't believe this was happening yet again." A Hard Decision Michael's squamous cell carcinoma, determined by the pathology report to be significantly influenced by the HPV virus, had metastasized to his lymph nodes on both sides of his neck, and his doctor said it could be stage four cancer. He remembers feeling like he was in quicksand, unsure if he should follow through with his move to Reno, or stay in the Bay Area for treatment. By now, it was early December 2020, and hospitals in the Bay Area and across the world were at limited capacity due to COVID-19. But, in what Michael describes as a positive twist of fate, the San Francisco ear, nose and throat provider he had seen about his biopsy results mentioned that he knew many providers in the oncology department at Renown, including Abhinand Peddada, MD. The San Francisco provider called Dr. Peddada's office with a referral, and Michael even remembers that Renown called him to hear more about his diagnosis before he even got the chance to call them "To be honest, I was feeling shut out in the Bay Area, and Dr. Peddada said he could help me expedite the treatment process," Michael said. "I finally felt a sense of relief." And so began Michael's 7-week chemoradiation cancer treatment program at Renown.
-
What Does It Mean to Participate in a Clinical Trial?
Participating in a clinical trial is voluntary and a personal choice. Clinical trials are research studies that involve people and are an important part of patient care. What is a clinical trial? Clinical trials are research studies that involve people, and they are an important part of patient care. There are several different types of clinical trials; some are designed to understand trends in a disease or identify better ways to diagnose a condition, while others determine if a new treatment is safe and works when treating, improving or preventing a health condition. There are over 400,000 clinical trials currently being conducted in the United States, and even more across the world. This includes health conditions such as heart failure, cancer, Parkinson’s Disease, respiratory conditions like COPD, common infections, cystic fibrosis, and many more. Clinical trials lead the healthcare industry to new discoveries that contribute to reliable and exact care, improving healthcare quality and saving lives. Clinical trials are conducted by a team of researchers, including doctors, pharmacists and clinical research coordinators. These research teams are highly skilled in their specialty areas, often providing traditional patient care and seeing research patients in the same day. These teams are responsible for making sure the clinical trial is completed correctly, and their patients are their top priority. Why should I consider participating in a clinical trial? Participating in a clinical trial is voluntary and a personal choice. There are many reasons why patients decide to get involved in clinical research. While many clinical trials are designed for patients who have a certain health condition, many studies also ask healthy volunteers to contribute in order to compare health outcomes. Clinical trials are also for patients at all different stages of their diagnosis. Depending on the specific study, the patient may receive access to a new cutting-edge treatment before it is widely available. When patients join a clinical trial, the research team becomes a health partner dedicated to their health and well-being. When patients join a clinical trial, they make an informed decision in their healthcare by weighing all available options in addition to routine treatments. Research participants know that they are contributing meaningfully and helping other patients like them. Where can I find more information about clinical trials at Renown Health? Renown Health’s mission is to make a genuine difference in the health and well-being of the communities we serve. Renown’s clinical trial portfolio offers leading care options to patients in northern Nevada, close to home, in a variety of specialties. Contact the Renown Clinical Research Office for more information on clinical trials available to you!
Read More About What Does It Mean to Participate in a Clinical Trial?
-
Eight Lessons from an MD-PhD Candidate at UNR Med
Meet newly minted Dr. Majid Khan, PhD., a native of Reno, and current MD-PhD candidate and first-generation medical student at the University of Nevada, Reno School of Medicine, who is on his way to a career as a Neurosurgery. While most graduate students often choose between pursuing a medical degree (MD) or a doctorate in philosophy (PhD), Majid has boldly picked both. He is one of three medical students currently in the UNR Med MD-PhD Program run by Dr. Caroline Cobine, PhD and Dr. Violeta Mutafova-Yambolieva, MD, PhD. "Research is not merely an act of sitting at a computer and reading papers – it's about answering the thought-provoking questions about things we come across on a daily basis. By critically evaluating these ideas we can implement solutions to enhance various aspects of the medical field and patient care with an overall goal of improving patient outcomes," Majid said. Majid recognizes that modern research extends beyond academia and holds significant value for hospitals. “Research contributes to improving patient outcomes. By reviewing the data from peer-reviewed research studies, medical professionals can be better prepared to deliver effective care following the most up-to-date guidelines and data,” he said. Majid's journey to pursuing his MD-PhD with a goal of becoming a physician-scientist-surgeon began following a summer in the PathMaker Cancer Research Program at the Huntsman Cancer Institute at the University of Utah. "It was by fate that I stumbled into this field – ever since I saw my first brain surgery, I haven’t been able to look back," Majid said. Here are some of the valuable lessons that Majid has learned along the way. 1. Beyond the 9 to 5, Embrace both 5 to 9s To avoid burnout and nurture personal passions, make your time spent outside of work and school intentional. Harness any free time to reconnect with friends, pursue hobbies and engage with mentors and mentees. 2. Collaboration is Key Work collectively with colleagues locally, nationwide and even worldwide. Cultivate environments to share knowledge and innovation, as well as wisdom, which will evidently lead to more impactful outcomes. 3. Shine a Spotlight on Your Colleagues Acknowledge and celebrate your colleagues in group settings when you notice something outstanding that they’ve said or done – it could anything big or small. Shining the spotlight onto those who are making positive changes within the hospital can inspire a beautiful culture of academic healthcare, which ultimately improves patient outcomes. 4. Redefine Mentorship Mentorship does not need to be confined to traditional frameworks. Seek out guidance in unexpected and untraditional places; sometimes, the most enlightening lessons and opportunities emerge from the most unlikely sources. 5. Diversify Your Experiences Embracing a diverse range of experiences enriches one's medical acumen. You never know when a seemingly unrelated job or experience will help in a scenario in your career. 6. Live By the Mamba Mentality Follow the late Kobe Bryant’s approach to life and work, the Mamba Mentality. This includes planning long-term goals, placing meaning in everything, striving for constant personal growth, following your passions and focusing on the process rather than the end goal. 7. Make Time for Your Loved Ones Don’t forget who helped you get to where you are in your life, specifically your family, friends, teachers and mentors. By making time for the most important and loving people in your life, you will be surrounded by positivity which will help propel you to new heights. 8. Plan your Next Five Moves We all have the ability to come from nothing and become something. Take the time to plan out everything and execute your moves with careful precision. Majid has plans to return to the Biggest Little City after completing Neurological Surgery Residency Program. If you would like to get in touch with Majid, please reach out to him via email at majidk@med.unr.edu.
Read More About Eight Lessons from an MD-PhD Candidate at UNR Med
-
Neurosciences Clinical Trials
Neurosciences Clinical Trial Opportunities
-
How the UNR Med Affiliation Impacts Renown's Clinical Research
The University of Nevada, Reno School of Medicine (UNR Med) and Renown Health affiliated in June of 2021. First Integrated Health System The University of Nevada, Reno School of Medicine (UNR Med) and Renown Health affiliated in June of 2021. This agreement established Nevada’s first integrated health system with missions in education, patient care, and clinical research. While there was extensive media coverage regarding the historic partnership in general, there is less information regarding the important impact to the community. In terms of clinical research, the potential benefits are endless. The School of Medicine has a very successful program in molecular bio sciences. That means they have a plethora of brilliant minds working on the smallest components of life related to body systems and disease. With the affiliation, there is a distinct opportunity to bridge some of these important findings happening on campus and translate them into care protocols for the patients seen in the community. Making connections between the cellular basics to the physical manifestations of conditions is a key opportunity of the affiliation. Additionally, cooperative funding models for projects that fit in the translational research bucket are available to these researchers. Renown and UNR Med are poised to provide potentially cutting-edge treatments with bench to bedside research. Expanding Clinical Trials Perhaps the most important outcome of the affiliation is access to novel treatments via clinical trials. In the coming years, Renown and UNR Med will work to expand their clinical trial portfolio in an effort to provide a clinical trial option for many diseases in addition to standard of care. This endeavor will allow patients to make an informed choice about their healthcare. With novel treatments available right here in Reno, people won’t have to travel to other research institutions for similar care. This eases a tremendous potential burden on patients and families alike navigating complex illnesses like cancer. The affiliation is exciting and provides many opportunities for clinicians (present and future) and the community. Renown's Clinical Research Office is thrilled to be a part of this historic time and are enthusiastic to communicate our successes and opportunities to the community. We sincerely look forward to contributing to the vision of “a healthy Nevada.”
Read More About How the UNR Med Affiliation Impacts Renown's Clinical Research
-
Discover Renown Health’s First Rheumatology Study
Renown Health is excited to announce participation in our first rheumatology clinical trial, marking a significant milestone in our commitment to advancing medical research and improving patient care. The study, led by rheumatologist and site-level Principal Investigator Dr. Kennedy Ukadike, focuses on evaluating the safety and efficacy of the experimental drug litifilimab in patients with systemic lupus erythematosus (SLE). This groundbreaking study is titled "A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study to Evaluate the Efficacy and Safety of Litifilimab (BIIB059) in Adult Participants With Active Systemic Lupus Erythematosus Receiving Background Nonbiologic Lupus Standard of Care." The study will focus on participants who have the disease and are already taking standard medications. The main goal of the study is to learn about the effect litifilimab has on lowering the activity of the disease. Participants will be randomized to receive either a high or low dose of the drug, or a placebo, with the treatment period lasting 52 weeks and a follow-up safety period of up to 24 weeks. Dr. Kennedy Ukadike shared his enthusiasm for the study: “This clinical trial represents a pivotal step in our efforts to better understand and treat systemic lupus erythematosus. We are hopeful that the findings will lead to more effective therapies and improved quality of life for our patients. “Launching our first rheumatology study is a testament to Renown’s dedication to pioneering research and delivering cutting-edge treatments to our community. This initiative not only enhances our research capabilities but also brings hope to patients and families affected by SLE,” said Kristen Gurnea, the Director of Clinical Research at Renown Health. Renown Health invites patients who are interested in joining this important study or learning about other clinical trial opportunities available to you to contact the Renown Office of Clinical Research (OCR) at Renown-CRD@renown.org or 775-982-3646. To contribute to Renown’s research initiatives, visit this link and select Renown Clinical Research.
Read More About Discover Renown Health’s First Rheumatology Study
-
Renown Children's Hospital Secures Crucial Funding in Support of Childhood Cancer Clinical Trials
Grant from St. Baldrick’s Foundation to continue funding research coordinator position at Renown Renown Children’s Hospital, the only dedicated children’s hospital in northern Nevada, has successfully secured a grant to fund its Research Coordinator position, a role that is critical in helping pediatric oncology patients navigate cancer clinical trials. The grant comes from the St. Baldrick’s Foundation, the largest charity funder of childhood cancer research grants, who announced the recipients sharing $1.2 million in infrastructure grants on Nov. 19, 2024. As a team member in the Renown Research Office, the grant-funded Research Coordinator is a key part of the research and clinical trials process, supporting study start-up, patient recruitment and enrollment, data collection, regulatory submissions and quality and compliance assurance. This role is closely aligned with the Children's Oncology Group (COG), the world's largest organization devoted exclusively to childhood cancer. Renown joined the COG as a member institution in 2021, allowing its youngest patients to have access to the latest medical advancements by participating in clinical trials. These studies assess state-of-the-art approaches for childhood cancer treatment, supportive care and survivorship. "Receiving this grant from the St. Baldrick’s Foundation is a step forward in our work to provide world class care for children battling cancer," said Brian Erling, MD, MBA, President and CEO, Renown Health. “We are deeply grateful to St. Baldrick’s for their unwavering commitment to childhood cancer research and their belief in the importance of improving outcomes for our youngest patients." “We are honored to support the vital infrastructure of pediatric cancer research across the U.S.,” said Kathleen Ruddy, CEO of the St. Baldrick’s Foundation in a press release. “This funding not only strengthens clinical trials essential to finding cures but also reinforces our commitment to ensuring that, even in times of hardship, children have access to the care and hope they deserve.” “As a COG member hospital, Renown Children’s Hospital joins over 200 institutions, including Stanford, University of California, San Francisco and The Children's Hospital of Philadelphia in this prestigious distinction,” says Kristina Deeter, MD, MBA, FAAP, Chair of Pediatrics (UNR Med) and Physician-in-Chief, Renown Children’s Hospital. “The COG is made up of more than 9,000 experts worldwide and has nearly 100 active clinical-translational trials at any given time. Through the clinical trials process, pediatric oncology patients at Renown Children’s Hospital can receive newly developed therapies; and their families can rest assured knowing they can receive outstanding cancer care right here in Reno.” "This funding ensures that our dedicated Research Coordinator can guide patients and families through the complexities of clinical trials, giving them access to the most advanced treatments without traveling far from home,” said Seema Rao, MD, Pediatric Oncologist at Renown Children’s and Principal Investigator for the COG program at Renown Health. “COG membership allows our young patients to access promising treatments in development without having to travel outside of the region. Our membership in this prestigious national organizations shows that Renown Children’s is providing care that meets or exceeds COG’s high standards.” Research from the COG has drastically transformed children’s cancer outcomes. Just 50 years ago, pediatric cancer was thought to be untreatable; today, it has a combined five-year survival rate of 80 percent. About Renown Health Renown Health is Nevada’s largest, not-for-profit integrated healthcare network serving Nevada, Lake Tahoe, and northeast California. With a diverse workforce of more than 7,500 employees, Renown has fostered a longstanding culture of excellence, determination, and innovation. The organization comprises a trauma center, two acute care hospitals, a children’s hospital, a rehabilitation hospital, a medical group and urgent care network, and the locally owned not-for-profit insurance company, Hometown Health. Renown is currently enrolling participants in a community-based genetic population health study, the Healthy Nevada Project®. For more information, visit renown.org. About Renown Clinical Research At Renown Health, our goal is to make it easy for patients to access clinical research as a care opportunity where patients can access a variety of standard care treatment options for their health condition or choose to participate in a clinical trial. To support pediatric cancer research in northern Nevada, indicate Renown Clinical Research on the donation form with Renown Health Foundation at renown.org/give. For more information about clinical trial opportunities available to you or to ask any questions, contact the Renown Clinical Research Office at Renown-CRD@renown.org or 775-982-3646.
-
Newly Expanded Clinical Research Center at UNR Med Fosters Collaboration and Research with Renown Health
Renown Health and the University of Nevada, Reno School of Medicine (UNR Med) are proud to announce a newly integrated and expanded research space called the Clinical Research Center (CRC). This space offers a dynamic physical location on the University of Nevada, Reno campus that supports the UNR Med and Renown Health research enterprise. "The partnership between Renown Health and UNR Med truly knows no bounds, and this Clinical Research Center is an incredible example of that endless possibility,” said Thomas Graf, MD, interim CEO of Renown Health. “This new space will only continue to expand our community’s access to clinical research as part of patient care while providing the necessary resources to engage our students and support a healthy Nevada.” This space’s capabilities include experienced staff with knowledge and skills in operationalizing FDA and non-FDA regulated clinical and translational research studies in a centralized 5,470-square foot research clinic housed in the Center for Molecular Medicine (CMM) at the University. This CRC space provides resources including: A centralized location next to the laboratory space that allows for strategic interdisciplinary collaboration between clinicians and basic scientists. Eleven private outpatient rooms for research clinic visits. Two blood draw stations. Physician consultation areas. Conference room for trial monitoring and consulting. Secure Investigational Product storage and preparation. Sample processing and storage, including countertop refrigerated centrifuges, 4°, -20° and -80° C refrigerators and freezers. Operations around clinical research are becoming more complex so growing clinical research in our community will require expertise and dedicated space where clinical research can be conducted in a learning environment first,” said Danielle Eaton, Director of Clinical Research with UNR Med and Renown Health. “This Clinical Research Center provides such space and experienced staff where these research studies can be successfully completed. The CRC provides a training environment for students, residents, faculty and clinical research professional work-force that will be needed to bring cutting edge diagnostics, therapeutics and preventatives to our community.” Meet the Team: Danielle Eaton, UNR Med Director of Clinical Research Kristen Gurnea, Renown Health Manager of Clinical Research Amber Emerson, UNR Med Project Manager Valerie Smith, UNR Med Center Administrative Manager Annie Beach-Hills, Gina Castro, Michelle Mejia and Amil Trujillo-King, UNR Med Study Coordinators Dr. John Westhoff, UNR Med Chair of Internal Medicine, Emergency Medicine physician Dr. Sean Kandel, UNR Med Associate Program Director for Resident Research, Associate Professor of Internal Medicine Dr. Amneet Rai, UNR Med Clinical research pharmacist Dr. Kellie Watkins, UNR Med Clinical Epidemiologist/Data Manager/Statistician As part of the affiliation between UNR Med and Renown Health, the Clinical Research Office is part of an integrated Office of Clinical Research, which allows both entities to collaborate on shared research program objectives. This effort allows colleagues to partner on clinical research, and to leverage bench-to-bedside research and delivery of leading-edge healthcare to northern Nevadans. About Renown Health Renown Health is the region’s largest, locally governed, not-for-profit integrated healthcare network serving Nevada, Lake Tahoe and northeast California. With a diverse workforce of more than 7,000 employees, Renown has fostered a longstanding culture of excellence, determination and innovation. The organization comprises a trauma center, two acute care hospitals, a children’s hospital, a rehabilitation hospital, a medical group and urgent care network, and the region’s largest, locally owned not-for-profit insurance company, Hometown Health. Renown is currently enrolling participants in the world’s largest community-based genetic population health study, the Healthy Nevada Project®. About UNR Med The University of Nevada, Reno School of Medicine (UNR Med), Nevada’s first public medical school, is a community-based, research-intensive medical school with a statewide vision for a healthy Nevada. Since 1969, UNR Med has trained more than 3,900 students, residents and fellows. UNR Med continues to improve the health and well-being of all Nevadans and their communities through excellence in student education, postgraduate training and clinical care, research with local, national and global impact and a culture of diversity and inclusion. For more information, visit med.unr.edu.
-
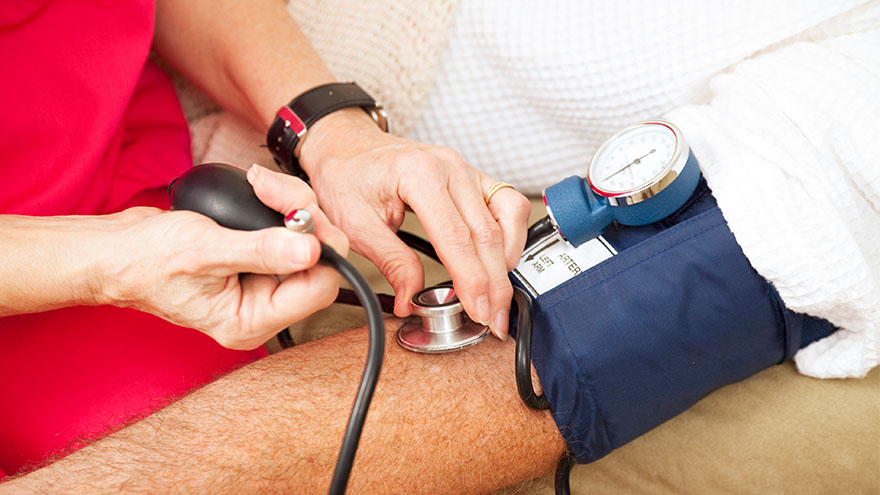
Managing Your Uncontrolled High Blood Pressure
Renown Health, the region's leading cardiology care provider, is offering a clinical trial for eligible patients struggling to control their high blood pressure. Nearly half of adults (119.9 million) in the United States have hypertension, or blood pressure that is higher than normal. Hypertension can put you at risk of other life-threatening disease, such as a heart attack or stroke. There are methods that cardiologists use to manage high blood pressure, but only 1 in 4 adults with hypertension (27.0 million) have their blood pressure under control.* Some patients with high blood pressure experience resistant hypertension, which does not respond well to multiple antihypertensive medications given at the same time. This means that there are many important opportunities for healthcare professionals to explore new ways to treat hypertension. At Renown Health, we lead the region in cardiology care with our technological expertise and patient-centered approach. That is why our cardiology team is partnering with the Renown Research Office to offer the RADIANCE Continued Access Protocol (RADIENCE CAP) clinical trial to eligible patients. RADIANCE CAP is a non-randomized study designed to allow for continued access to ultrasound renal denervation therapy via the Paradise System, and to allow for the on-going collection of safety and effectiveness data in patients with uncontrolled hypertension despite the prescription of antihypertensive medications. The body’s complex communication system between the brain, heart and kidneys can sometimes become overactive, increasing your blood pressure through messages in the nervous system. Renal denervation is a minimally-invasive procedure which reduces activity from the nerves in your kidneys to lower blood pressure. This is the third in a series of renal denervation clinical trials Renown Health has offered to patients with resistant hypertension over the last several years, with over 40 local participants. “All the participants that I have had the pleasure to work with on these studies are very excited and grateful to have this option for helping control their blood pressure” states Lisa English, Lead Clinical Research Coordinator for Cardiology studies at Renown Health. “I love getting to know each one of them and helping on their healthcare journey. We have an amazing team of providers and staff at Renown that go out of their way to make patients experiences positive and the studies successful.” Dr. Michael Bloch, Cardiologist and Principal Investigator for the RADIANCE CAP study at Renown Health’s Institute for Heart and Vascular Health adds, “Despite lifestyle modifications like diet and exercise and the widespread availability of effective and well-tolerated medications, approximately 50% of all people with hypertension have inadequate blood pressure control putting them at risk for stroke, heart failure and kidney disease. As a one-time durable procedure, renal denervation with the Paradise endovascular system from ReCor Medical, Inc. may help millions of patients improve their blood pressure control without necessarily needing to increase their medications.” Our teams of expert providers and researchers are here to support you on your healthcare journey. Talk to your provider about the RADIANCE CAP clinical trial at your next appointment to see if participation may be right for you.
Read More About Managing Your Uncontrolled High Blood Pressure
-
Getting to the HEART of Research
In February, we think about hearts not just in honor of Valentine’s Day but because it is American Heart Association Month. This is a great reminder to focus on our personal cardiovascular health. Renown Health helps patients think about their heart health with our world-class providers and cutting-edge treatments through our Cardiovascular Clinical Trials. “Research serves a vital role in the future care of cardiovascular diseases. Being involved in research will help our medical community to further discover new treatment plans in our quest for life preservation and extension,” Dr. Thomas To, Cardiologist and Researcher at Renown Health. For example, let’s talk about atherosclerosis. When our hearts are healthy, they are a strong muscle that pumps our oxygen-rich blood through our coronary arteries. Over time, cholesterol and fats can build up in our arteries. This is a condition known as atherosclerosis. This type of plaque buildup in the arteries can lead to a heart attack or stroke if not properly managed. If you are experiencing chest pain or discomfort, shortness of breath or pain in areas of the upper body, these can be the warning signs of a heart attack, and you should call 911. One contributing factor to atherosclerosis is elevated lipoprotein(a) levels and the accumulation of cholesterol in the arteries, which increases the likelihood of a heart attack or stroke. Lipoprotein(a) is tested separately from the standard panel that is completed for cholesterol management, and while your total cholesterol levels may be in a healthy range, lipoprotein(a) levels can still be elevated. "Increasingly we are realizing that lipoprotein(a) levels can be used as an important assessment in more carefully delineating an individual's risk of future cardiovascular events and treatment targets" said Dr. Michael Bloch, Lipid Specialist and Researcher at Renown Institute for Heart and Vascular Health. While it is clear that elevated lipoprotein(a) contributes to atherosclerosis, there are currently no approved medications for reducing cardiovascular disease risk through reducing lipoprotein(a) levels. This is why Renown Health’s Research Office is proud to offer a phase III clinical trial, called the OCEAN(a) study, to our patients with elevated lipoprotein(a) levels as a care option for management of their heart disease risk. Our teams of expert providers and researchers are here to support you on your healthcare journey. “I am thrilled to be able to be part of this study and bring opportunities like this to our patients. The highlight of my day is getting to hear life stories from my patients during our study visits,” Lisa Preciado, Primary Clinical Research Coordinator for the OCEAN(a) study said. Join us in raising awareness around American Heart Month by talking to your provider about lipoprotein(a) at your next appointment. At Renown Health, our goal is to make it easy for patients to access clinical research as a care opportunity where patients can access a variety of standard care treatment options for their health condition or choose to participate in a clinical trial. For more information about clinical trial opportunities available to you or to ask any questions, contact the Renown Research Office at Renown-CRD@renown.org or 775-982-3646.
-
Keeping Research Close to Northern Nevada
Clinical research provides agency for our patients navigating a scary diagnosis, and the field has never been stronger in northern Nevada. This strength is thanks in part to the Affiliate Clinical Research Office (ACRO) formed by the 2021 affiliation between Renown Health and the University of Nevada, Reno School of Medicine. Since its creation, the ACRO team has been busy ensuring that community members have access to the latest care options and exceptional experiences as participants in both research and their healthcare. Here are just a few things that set this office apart from the rest. 1. A focus on engagement In 2022, the ARCO team focused on promoting a research culture with patients, clinicians, residents and students by intentionally engaging with healthcare providers, department administrators, internal research team members and leadership. They educated the community with learning materials that emphasized the importance of doing research. This team also worked with front-line staff to raise awareness and excitement about the clinical research options available for Renown Health patients. 2. Meaningful partnerships The most impactful partnership to date is between Renown Health and UNR Med. By identifying opportunities and leveraging resources across institutions, we have maximized our impact and built a solid and sustainable foundation. This gives the people of northern Nevada greater access to new interventions or novel treatments. This team is also investing in the community and national partnerships to provide training opportunities for our research staff and learning opportunities for our medical students. 3. Novel treatments across many disease areas Our research study offerings must reflect the healthcare needs of our community and the expertise of practicing clinicians. The department has over 80 active studies in neurology, pulmonology, oncology, cardiology, pediatrics and disease prevention. The ARCRO team strives to expand care opportunities to allow our community members to stay close to home when seeking care. This year, they will continue exploring our community's unmet healthcare needs by bringing new treatment options to the greater Reno area.